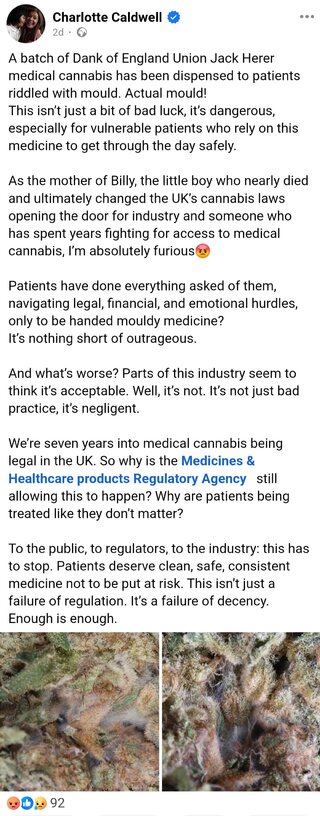
Dear Patient,
A thorough investigation has been conducted, including review of the necessary tests required for the product to meet import requirements.
The investigation has concluded that the product met the quality requirements set by regulators
Please see below summary from the investigation:
Thank you for your patience while we completed the investigation into the product complaint for batch A299918.
We have reviewed the images provided by the patient, assessed the retention samples, and examined the Certificate of Analysis for this batch. No presence of mold or other foreign matter was detected, and all results fell within the approved specifications.
Additionally, the full batch manufacturing documentation has been reviewed. No deviations or signs of contamination were observed during production, in-process checks, or final quality assessments. All procedures were conducted in line with Good Manufacturing Practice standards, and the batch is confirmed to meet the required quality, safety, and efficacy criteria.
Given these findings, the supplier recommend that the patient speak with their prescriber for advice on whether to continue treatment.
We do want to apologies again that the product did not meet your expectations. We also want to reassure you that we continue to log and review quality concerns and that we use these to learn from and improve the service and product that we deliver to our patients.
Kind Regards,
Montu